| ||||
Chemistry Basics & the Periodic Table
Practice Test Questions
from Science Prof Online
Atomic Theory & Periodic Table Test Qus
Atomic Theory & Periodic Table
Free review questions to help students better understand Atomic Theory & the Periodic Table.
 | ||||||
SPO VIRTUAL CLASSROOMS
The following questions, from the Virtual Cell Biology Classroom, are designed to help students better understand Atomic Theory and the Periodic Table. All questions are based on materials that can be found on the Atomic Theory & Periodic Table Lecture Main Page.
1. Which is simplest?
a. molecule
b. atom
c. elephant
d. compound
e. synthesis
2. How is an isotope different than a normal atom?
a. different number of electrons
b. different number of protons
c.different number of quarks
d. different number of neutrons
3. The ____ charge of a proton is balanced by the ____ charge of a(n) ____.
a. negative, positive, electron
b. positive, neutral, neutron
c. negative, negative, electron
d.positive, negative, electron
4. If an element's atomic number is 6, its mass number is ________.
a. 3 b. 6 c. 8 d. 12 e. 18
5. The maximum number of valence electrons an atom can have is ____.
a. 2 b. 4 c. 8 d. 12 e. 18
6. You can determine the number of electron orbitals an atom has by looking at its _______.
a. atomic number b. group number
c. mass number d. period number
10. What is the maximum number of electrons an atom can have on its innermost orbital?
a. 2 b. 4 c. 8 d. 12 e. 18
Page last updated: 5/2016
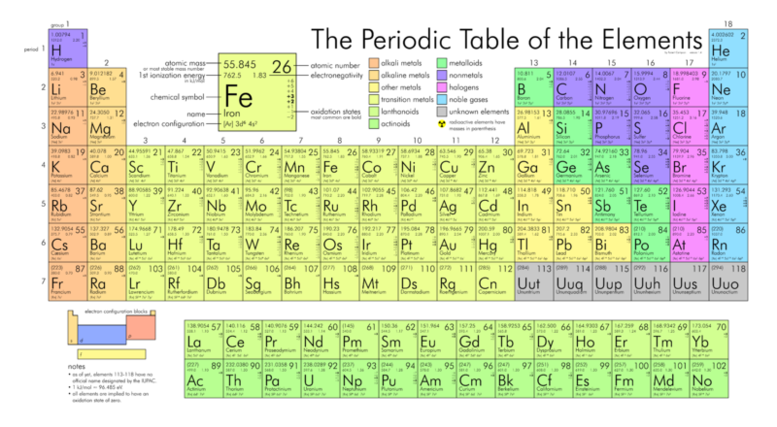
Click here for a printable, detailed periodic table.
You have free access to a large collection of materials used in a college-level introductory Cell Biology Course. The Virtual Cell Biology Classroom provides a wide range of free educational resources including Power Point Lectures, Study Guides, Review Questions and Practice Test Questions.
 | ||||||
SPO offers free PowerPoint lectures, sample test
questions, review questions and assignments on many different science topics.
Below are links to those that relate to chemistry:
7. If the symbol for a chemical element contains two letters, like the “Na” of sodium, we know that:
a. It is a very large atom.
b. It is the most common atom that begins with the letter N.
c. It is a very small atom.
d. It is not the most common element to begin with the letter N.
8. How many valence electrons does Sodium (Na) have? (Check your periodic table.)
a. 1 b. 2 c. 3 d. 4 e. 8
9. Changing the number of neutrons in an atom makes it a(n) ________. Changing the number of electrons alters an atoms ________. But changing the number of protons in an atom would make it a(n) ________.
a. ion, atomic number, isotope
b. isotope, charge, different element
c. different element, mass number, isotope
d. positive atom, atomic mass, ion
e. grouchy bear, swag, total badass
10. What is the maximum number of electrons an atom can have on its innermost orbital?
a. 2 b. 4 c. 8 d. 12 e. 18